EXECUTIVE SUMMARY:
SMELL-SEEING
Array based vapor sensing has emerged as a powerful approach toward the detection of chemically diverse analytes. Based on cross-responsive sensor elements, these systems aim to produce composite responses unique to each odorant, in a fashion similar to the mammalian olfactory system. We have invented the colorimetric sensor array [Rakow, N. A.; Suslick, K. S. "A Colorimetric Sensor Array for Odour Visualization" Nature, 2000, 406, 710-714.; Suslick, K. S.; Rakow, N. A. "Colorimetric Artificial Nose Having an Array of Dyes and Method for Artificial Olfaction" U.S. Patent 6,368,558; April 9, 2002. Suslick, K. S.; Rakow, N. A.; Sen, A. "Colorimetric Artificial Nose Having an Array of Dyes and Method for Artificial Olfaction: Shape Selective Sensors" U.S. Patent 6,495,102 B1; Dec. 17, 2002. ]. This "optoelectronic nose"
has proved to be an extremely effective approach to the detection, identification, and quantification of odorants of all kinds and has received widespread publicity and recently led to the founding of iSense, a start-up company located in Palo Alto.
"Smell-Seeing"
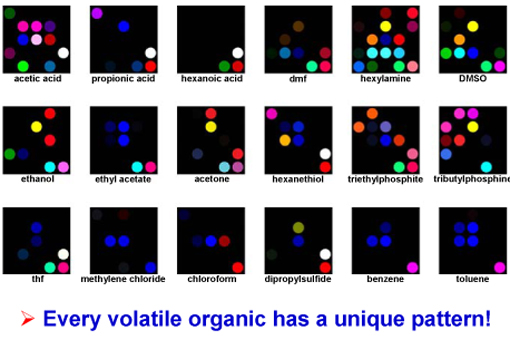
As inorganic chemists have long realized, compounds that bind to metal ions are nearly always highly odiferous. For this reason, metal-containing dyes, such as metalloporphyrins, provide a way of reporting the presence of odors by changes in color. We first started our sensor by using a two-dimensional display of metalloporphyrins as sensor elements for the visual identification of a wide range of olfactants (including alcohols, amines, ethers, phosphines, phosphites, thioethers, and thiols) and even weakly-ligating solvent vapors (arenes, halocarbons, and ketones). The solid-state color changes are similar to those known for metal ligation in solution.
We expanded from this simple array to include a total of 36 chemically responsive colorants in a printable nanoporous formulation for detection and identification of volatile organic compounds (VOCs). These are inexpensive, disposable sensor arrays based on equilibrium interactions of analytes with metalloporphyrins and other chemically responsive dyes. As with human olfaction, our colorimetric sensor arrays use a large number of cross-reactive sensors that probe a wide range of chemical properties. By digitally monitoring the change in color of each dye in the array, we have a quantitative measure of a composite response to VOCs. Chemometric pattern recognition is extremely powerful with these arrays because of their very high dimensionality.
The recent goals in my research group have been to extend this technology to biomedical applications, with specific aims focusing (1) on the development of a personal chemical dosimeter for the detection, identification, and quantification of environmental and workplace VOCs and (2) on medical diagnosis tools based on breath analysis. The sensitivity of our arrays permits rapid detection of very low levels of most volatile toxicants. This is a translational technology that should find substantial use in workplace monitoring of chemical exposure and in the near future to the development of new point-of-care tools for medical diagnosis and screening (e.g., lung cancer, sinusitus, liver dysfunction, ...). Our efforts in these areas should lead (1) to fundamental advances in sensor development for molecular recognition and biomedical applications of such sensor arrays, (2) development and refinement of technology for the rapid and continuous identification and quantitation of volatile chemical toxicants, and most importantly, (3) prototyping of an extremely portable device for the assessment of personal VOC exposure
We have made excellent progress on the design of the colorimetric array, production of a handheld prototype reader/analyzer for the array, testing of the array against toxic industrial chemicals (TICs), and design of a new wearable sized reader/analyzer. As recently published in in Nature Chem. and in Chem. Commun., we have tested our current colorimetric sensor array against a battery of 20 high priority TICs at their immediately dangerous to life or health concentrations (IDLH) and Permissible Exposure Limits (PEL) and even down to ~5% of PEL. We are able to distinguish among all of these TICs at permissible exposure limit (PEL) concentrations without errors.
In other work, we have made the connection complete by finding a conserved metal ion binding site in the mammalian olfactory recetors and proposed the first molecular mechanism of action of the olfactory receptors. Wang, J.; Luthey-Schulten, Z.; Suslick, K. S. "Is the Olfactory Receptor A Metalloprotein?" Proc. Natl. Acad. Sci. U.S.A., 2003, 100, 3035-3039.
Some of our Papers on Smell-Seeing, Olfaction, and Some Related Topics:
- Rakow, N. A.; Suslick, K. S. "A Colorimetric Sensor Array for Odour Visualization" Nature, 2000, 406, 710-714.
- Sen, A; Suslick, K. S. "Shape-Selective Discrimination of Small Organic Molecules" J. Am. Chem. Soc., 2000, 122, 11565-11566.
- Zimmerman, S. C.; Wendland, M. S.; Rakow, N. A.; Zharov, I.; Suslick, K. S. "Synthetic Hosts by Monomolecular Imprinting Inside Dendrimers" Nature 2002, 418, 399-403.
- Wang, J.; Luthey-Schulten, Z.; Suslick, K. S. "Is the Olfactory Receptor A Metalloprotein?" Proc. Natl. Acad. Sci. U.S.A., 2003, 100, 3035-3039.
- Zimmerman, S. C.; Zharov, I.; Wendland, M. S.; Rakow,N. A.; Suslick, K.S. "Molecular Imprinting Inside Dendrimers" J. Am. Chem. Soc. 2003, 125, 13504 - 13518.
- Suslick, K. S.; Rakow, N. A.; Sen, A. "Colorimetric sensor arrays for molecular recognition" Tetrahedron, 2004, 60, 11133-11138.
- Rakow, N. A.; Sen, A., Janzen, M.C.; Ponder, J. B.; Suslick, K. S. "Molecular Recognition and Discrimination of Amines with a Colorimetric Array" Angew. Chem. Int. Ed. 2005, 44, 4528-4532.
- Zhang, C; Suslick, K. S. "A Colorimetric Sensor Array for Organics in Water", J. Am. Chem. Soc. 2005, 127, 11548-11549.
- Janzen, M. C.; Ponder, J. B.; Bailey, D. P.; Ingison, C. K.; Suslick, K. S. "Colorimetric Sensor Arrays for Volatile Organic Compounds" Anal. Chem. 2006, 78, 3591-3600.
- Zhang, C.; Bailey, D. P.; Suslick, K. S. "Colorimetric Sensor Arrays for the Analysis of Beers: A Feasibility Study" J. Agric. Food Chem., 2006, 54, 4925-4931.
- Zhang, C.; Suslick, K. S. "Colorimetric Sensor Arrays for Soft Drink Analysis" J. Agric. Food Chem., 2007, 55, 237-242.
- Bang, J. H.; Lim, S. H.; Park, E.; Suslick, K. S. "Chemically Responsive Nanoporous Pigments: Colorimetric Sensor Arrays and the Identification of Aliphatic Amines" Langmuir 2008, 24, 13168-13172.
- Musto, C. J.; Lim, S.H.; Suslick, K. S. "Colorimetric Detection and Identification of Natural and Artificial Sweeteners" Anal. Chem. 2009, 81, 6526-6533.
- Lim, S. H.; Feng, L.; Kemling, J. W.; Musto, C. J.; Suslick, K. S. "An Optoelectronic Nose for Detection of Toxic Gases" Nature Chemistry, 2009, 1,
562-567.
- Suslick, B. A.; Feng,L.; Suslick, K. S. "Discrimination of Complex Mixtures by a Colorimetric Sensor Array: Coffee Aromas" Anal. Chem., 2010, 82, 2067-2073.
- Feng, L.; Musto, C.J.; Kemling, J. W.; Lim, S.H.; Suslick, K. S. "A Colorimetric Sensor Array for Identification of Toxic Gases below Permissible Exposure Limits" Chem. Commun., 2010, 46, 2037-2039.
- Feng, L.; Musto, C.J.; Suslick, K. S. "A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde" J. Am. Chem. Soc., 2010, 132, 4046-4047.
- Kemling, J. W.; Qavi, A. J.; Bailey, R. C.; Suslick, K. S. "Nanostructured Substrates for Optical Sensing" J. Phys. Chem. Lett. 2011, 2, 2934-2944. Invited review with cover.
- Kemling, J. W.; Suslick, K. S. "Nanoscale Porosity in Pigments for Chemical Sensing" Nanoscale 2011, 3, 1971-1973.
- Carey, J. R.; Suslick, K. S.; Hulkower, K. I.; Imlay, J. A.; Imlay, K. R. C.; Ingison, C. K.; Ponder, J. B.; Sen, A.; Wittrig, A. E. "Rapid Identification of Bacteria with a Disposable Colorimetric Sensor Array" J. Am. Chem. Soc. 2011, 133, 7571-7576.
- Lin, H.; Jang, M.; Suslick, K. S. "Preoxidation for Colorimetric Sensor Array Detection of VOCs"
J. Am. Chem. Soc. 2011, 133, 16786-16789.
- Mazzone, P. J.; Wang, X.-F.; Xu, Y.; Mekhail, T.; Beukemann, M. C.; Na, J.; Kemling, J. W.; Suslick, K. S.; Sasidhar, M. "Exhaled Breath Analysis with a Colorimetric Sensor Array for the Identification and Characterization of Lung Cancer" J. Thoracic Oncology 2012, 7, 137-142.
- Mahmoudi, M.; Suslick, K. S. "Protein Fibrillation and the Olfactory System: Speculations on Their Linkage" Trends in Biotech. 2012, 30, 609-610.
- Askim, J. R.; Mahmoudi, M.; Suslick, K. S. "Optical sensor arrays for chemical sensing: the optoelectronic nose" Chem. Soc. Rev. 2013, 42, 8649 - 8682. With cover.
- LaGasse, M. K.; Rankin, J. M.; Askim, J. R.; Suslick, K. S. "Colorimetric
sensor arrays: Interplay of geometry, substrate and immobilization" Sensors
& Actuators B-Chem. 2014, 197, 116-122.
- Zhang, Y.; Askim, J. R.; Zhong, W.; Orlean, P.; Suslick, K. S. "Identification
of pathogenic fungi with an optoelectronic nose" Analyst, 2014, 139, 1922-1928. DOI: 10.1039/C3AN02112B
- Rankin, J. M.; Suslick, K. S. "The development of a disposable gas chromatography microcolumn"
Chem. Commun. 2015, 51, 8920-8923. DOI: 10.1039/c4cc09915j
- Zhong, X.; Suslick, K. S.; Zhong, W. "Tensor Sufficient Dimension Reduction" WIREs Comput. Stat. 2015, 7, 178-184. DOI: 10.1002/wics.1350.
- Li, Z.; Jang, M.; Askim, J. R.; Suslick, K. S. "Identification of accelerants, fuels and post-combustion residues using a colorimetric sensor array" Analyst 2015, 140, 5929-5935. DOI: 10.1039/c5an00806a
- Li, Z.; Bassett, W. P.; Askim, J. R.; Suslick, K. S. "Differentiation among peroxide explosives with an optoelectronic nose" Chem. Commun. 2015, 51, 15312-15315. DOI: 10.1039/C5CC06221G
- Askim, J. R.; Suslick, K. S. "Hand-Held Reader for Colorimetric Sensor Arrays" Anal. Chem. 2015, 87, 7810-7816. DOI: 10.1021/acs.analchem.5b01499
- Askim, J. R.; Li, Z.; LaGasse, M. K.; Rankin, J. M.; Suslick, K. S. "An optoelectronic nose for identification of explosives" Chem. Sci., 2016, 7, 199-206. DOI: 10.1039/c5sc02632f
- Mahmoudi, M.; Lohse, S. E.; Murphy, C. J.; Suslick, K. S. "Identification of Nanoparticles with a Colorimetric Sensor Array" ACS Sensors, 2016, 1(1), 17-21. DOI: 10.1021/acssensors.5b00014
- Li, Z.; Li, H.; LaGasse, M. K.; Suslick, K. S. "Rapid Quantification of Trimethylamine" Anal. Chem., 2016, 88, 5615-5620. DOI: 10.1021/acs.analchem.6b01170
- Li, Z.; Suslick, K. S. "Portable Optoelectronic nose for Monitoring Meat Freshness" ACS Sensors, 2016, 1, 1330-1335. DOI: 10.1021/acssensors.6b00492

|
|








